Grants and MSOE Contracts Policies and Procedures
Grant Procedures & Getting Started Checklist
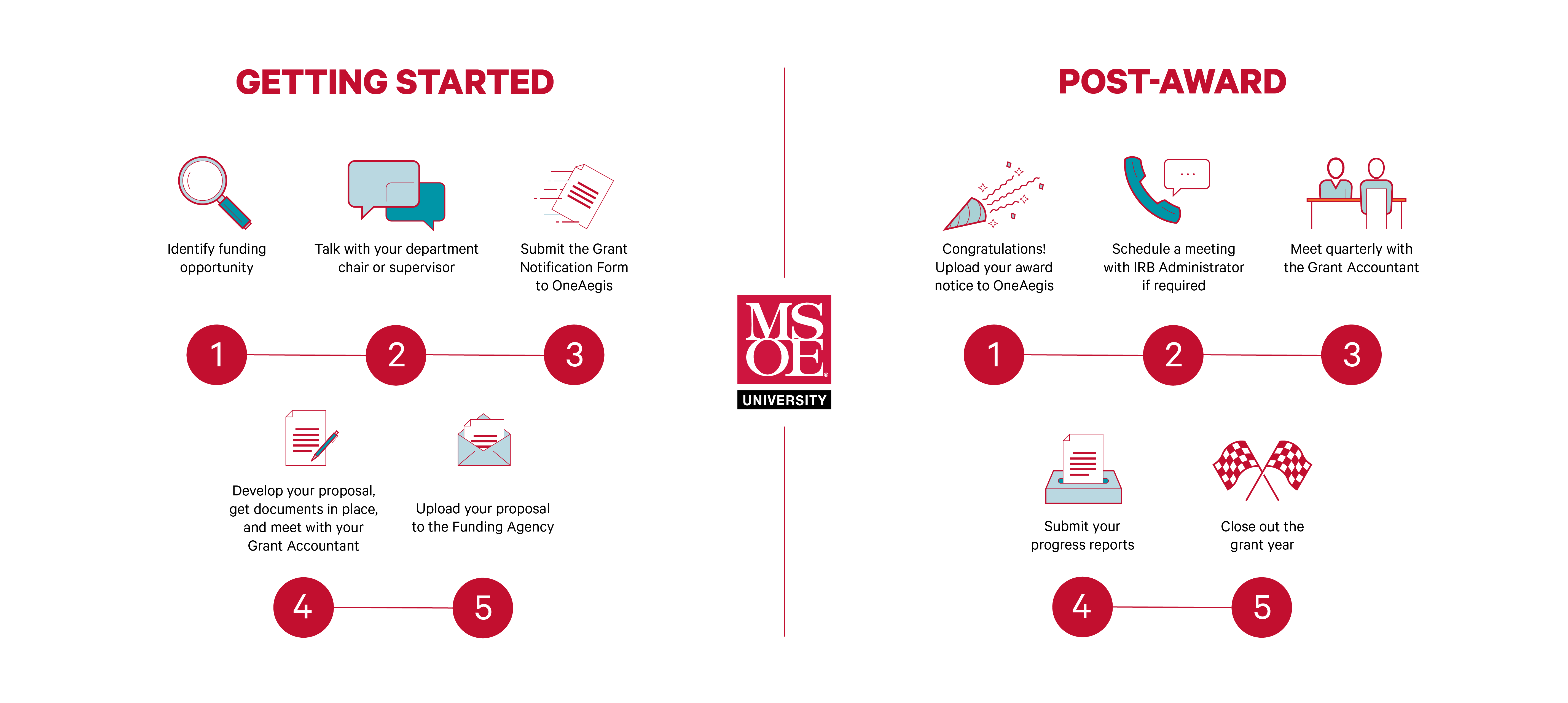
Pre-Award
- Identify Funding Opportunity
- Talk with your Department Chair or Supervisor
- Make sure to have a conversation with your department chair or supervisor early in the process. Frequent communication about new grants will help us coordinate efforts across departments and make sure you get connected with others who have developed similar projects or applied to the same funding agency
- Timeline: 2+ months prior to sponsor deadline
- Submit the Grant Notification Form
- This form will serve as a prompt for the team to formally begin the pre-award process inclusive of budget, CITI Program training certification by research team members for Responsible Conduct of Research and Human Subjects Research, IRB oversight for human subject participation, and other considerations. This is done within OneAegis.
- Timeline: 1 month prior to sponsor deadline
- Develop your Proposal, Get Documents in Place, and Meet with your Grant Accountant
- After your form is reviewed, MSOE’s Grant Accountant will reach out to you to begin coordinating your budget. At this time, researchers should also be collaborating with peers to produce the narrative portions of the application
- Timeline: 1-2 months prior to sponsor deadline
- Submit your Proposal to the Funding Agency
- Make sure to wait for internal approval from the Dean of Applied Research or Vice President of Academics before submitting your proposal. Please note that for some sponsors, like NIH and NSF, MSOE’s Authorized Organizational Representative (AOR) will be required to perform the final submission to the sponsor
- Timeline: 1-3 days prior to sponsor deadline
Post-Award
- Congratulations! Send your Award Notice to the Grant Accountant
- The Grant Accountant will retain a copy and also reconcile any changes to start dates, budget figures, and other items, as required. The GA will reach out to you if a meeting is required to further coordinate these materials
- Timeline: Immediately after award notice is received
- Schedule a Meeting with IRB Administrator, if required
- Reach out to MSOE’s IRB Administrator to finalize your project’s protocol package, if human subject participants will be involved with your research
- Timeline: Immediately after award notice is received
- Meet Quarterly with the Grant Accountant
- Quarterly meetings will help both parties to understand the project’s timeline in relation to financial requirements
- Timeline: Throughout the grant year
- Submit your Progress Reports
- Periodic progress reports are often required by the funding agency. Principal Investigator’s (PI’s) are required to ensure that these are prepared and submitted in a timely manner
- Timeline: Throughout the grant year, often quarterly
- Close Out the Grant Year
- Take the opportunity in the closing months of each budget year to meet with your Grant Accountant to ensure that total expenditures recorded to-date, capture an accurate representation of the grant as a whole. For example, are there any missing charges? This is an excellent time to coordinate and communicate any unrecorded expenses to be charged against the account before the end of the period. This will help you transition smoothly into the next period and ultimately to close the grant project as a whole
- If human subject participants are involved in the study
- Submit Continuing Review Form to MSOE IRB, as required for your project
- Submit Study Completion Form to MSOE IRB at the conclusion of the project
- Timeline: 1 month prior to budget year end date
Grant Policies
Drug-Free Workplace
Equal Opportunity Employer / Educator
Conflict of Interest Policy
Financial Conflict of Interest Policy
Research with Human Participants
Cost Allocation Policy
HIPAA
The Department of Health and Human Services has provided some basic Federal guidelines as to the usage and disclosure of private medical information. In the process of managing grants, principle investigators are made aware of this legislation and its possible impact on their grant project. View the NIH's Booklet for Research regarding HIPAA.
FERPA
The Family Educational Rights and Privacy Act (FERPA) (20 U.S.C. § 1232g; 34 CFR Part 99) is a Federal law that protects the privacy of student education records. The law applies to all schools that receive funds under an applicable program of the U.S. Department of Education, as does MSOE. Principle Investigators are encouraged to read through the synopsis of these guidelines on the U.S. Department of Education's web page and think about how it may apply to how they use or disclose student educational information in the activities of their grant project.
Federal Acquisition Regulations
The Federal Acquisition Regulations (FAR) governs all federal procurement for goods and services. In some instances, individual agencies have amended the FAR to meet their agency needs.
